Sirolimus-eluting stent
Biodegradable polymer matrix
The New Generation of Drug-Eluting Stent
Biodegradable polymer matrix
THE EFFECTIVE COMBINATION
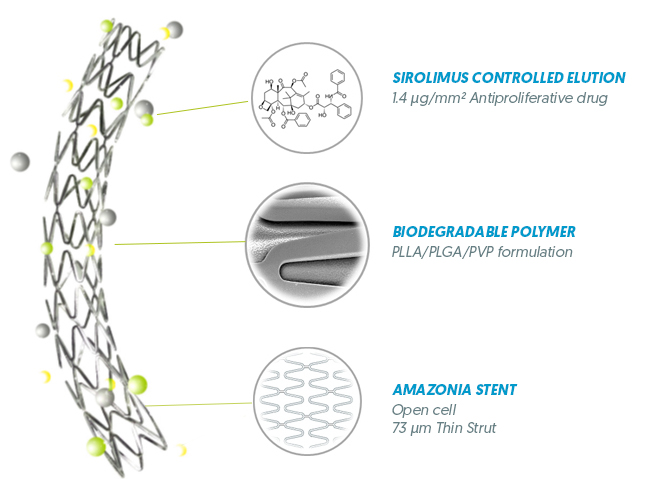
AMAZONIA STENT PLATFORM
Unique Proprietary Thin Strut Cobalt-Chromium stent
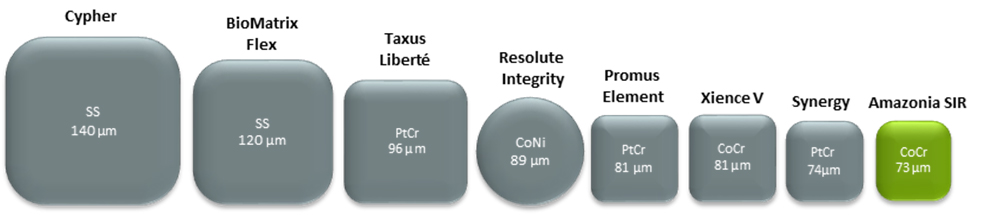
homogeneous scaffolding

Flexibility and Larger Contact Surface
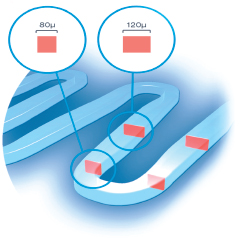
Reliable Stent Delivery System
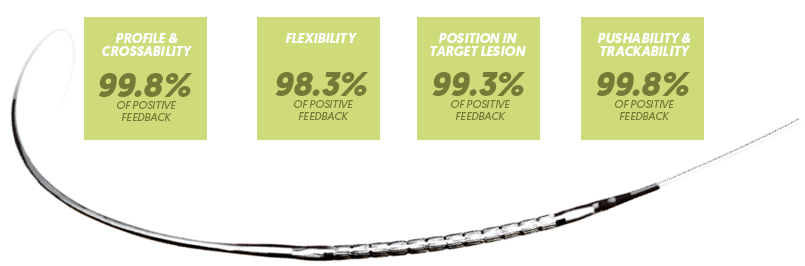
AMAZONIA Stent Delivery System Physicians feedback: 450 cases *
SIROLIMUS CONTROLLED ELUTION
- Antiproliferative action
- Reduction of inflammatory response
- 1.4 µg / mm² of Abluminal Stent surface
- 85% eluted at 48 days (in average*)
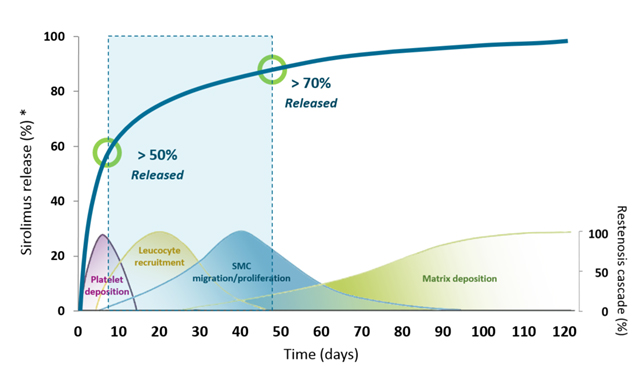
Elution Profile window perfectly adapted to prevent natural adverse effects of healing process
BIODEGRADABLE POLYMER
- Simultaneous polymer biodegradation and drug elution
- Nearly complete resorption expected within 180 days*
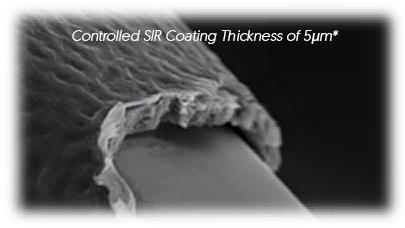
-
Protective layer to prevent initial
burst effect of Sirolimus - Fully degraded and metabolized
-
Complete reversion to BMS expected
within 180 days*
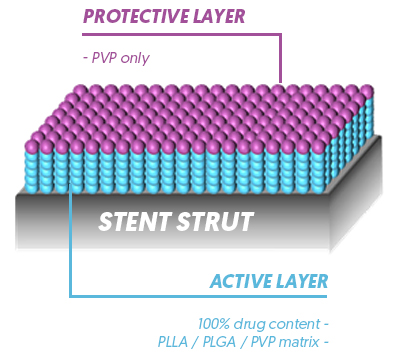
Abluminal Coating
- Combination with Thin Strut design for faster healing
- Limited luminal polymer exposure
- Reduced systemic exposure
- Reduced risk of delayed healing
- Early BMS-like endothelial coverage expected
PRE-CLINICAL EVALUATION
- Safety and healing performance verified
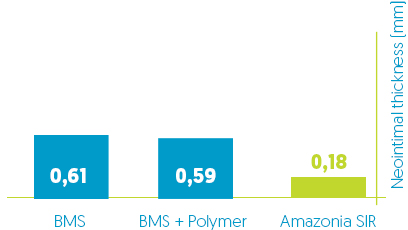
Comparative evaluation of safety and healing performance in porcine model *:
Amazonia SIR vs BMS vs BMS + Polymer
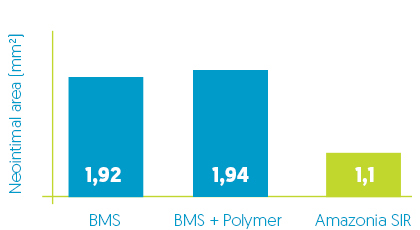
Neo-intimal thickness reduction*
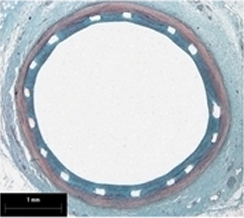
Histological section at 90 days
after Amazonia SIR implantation *
- Absence of inflammatory response
- Nearly complete polymer absorption
Clinical data
- Extensive Clinical Program for safety and performance demonstration
Ongoing Amazonia SIR e-Registry
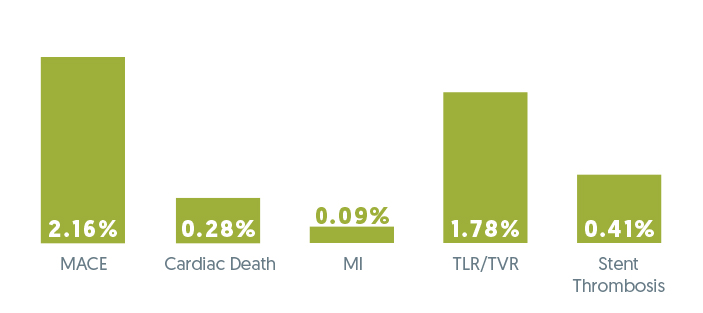
Safety & Performances demonstrated
- Low cumulative MACE rate at 12 months
- No evidence of Late Stent Thrombosis
- Low rate of cardiac events
Cumulative Clinical Events at 12 Months (%)
Clinical case
Patient: 79 years, male, chronic total occlusion.
Complete occlusion of the LCx treated with a 2.75x12 mm Amazonia SIR Sirolimus-eluting stent.
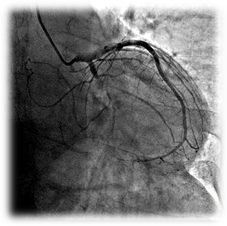
Pre-procedure
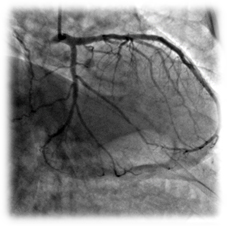
Post-procedure
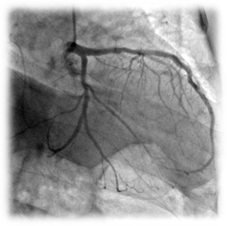
9 months post-procedure
Specifications
Device technical specification
The Amazonia SIR Stent is indicated for treatment of patients with stenosis in Coronary Arteries. It is indicated for improving the coronary luminal diameter in patients with symptomatic ischemic disease due to lesions of length ≤ 40 mm in native coronary arteries with a reference diameter from 2.25mm to 4.00mm.
| Drug | Sirolimus |
|---|---|
| Polymer | Biodegradable matrix |
| Coating repartition | Abluminal |
| Dosage | 1,4 µg/mm² |
| Stent Material | Cobalt Chromium Alloy L605 |
| Stent thickness | 73µm (+5µm drug coating) |
| Length | From 8 to 40 mm |
| Diameters | From 2.25 to 4.00 mm |
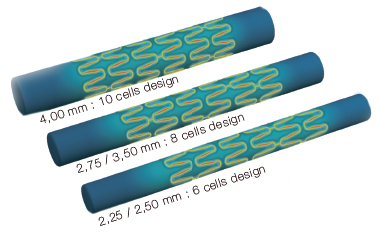
| Number of cells |
6 cells for Ø2.25 - 2.50 mm 8 cells for Ø2.75 to 3.50 mm 10 cells for Ø4.00 mm |
|---|---|
| Metal to artery ratio | From 14 to 16% |
| Nominal pressure | 8 atm |
| Rated Burst Pressure | 14 atm |
| Guidewire compatibility | 0.014" |
| Guiding catheter compatibility | 5F |
Ordering information
| Length (mm) | Diameter (mm) | |||||
|---|---|---|---|---|---|---|
| 2.25 | 2.50 | 2.75 | 3.00 | 3.50 | 4.00 | |
| 8 | AMSIR001 | AMSIR002 | AMSIR003 | AMSIR004 | AMSIR005 | AMSIR006 |
| 12 | AMSIR007 | AMSIR008 | AMSIR009 | AMSIR010 | AMSIR011 | AMSIR012 |
| 16 | AMSIR013 | AMSIR014 | AMSIR015 | AMSIR016 | AMSIR017 | AMSIR018 |
| 20 | AMSIR019 | AMSIR020 | AMSIR021 | AMSIR022 | AMSIR023 | AMSIR024 |
| 24 | AMSIR025 | AMSIR026 | AMSIR027 | AMSIR028 | AMSIR029 | AMSIR030 |
| 28 | AMSIR031 | AMSIR032 | AMSIR033 | AMSIR034 | AMSIR035 | AMSIR036 |
| 32 | AMSIR037 | AMSIR038 | AMSIR039 | AMSIR040 | AMSIR041 | AMSIR042 |
| 36 | AMSIR043 | AMSIR044 | AMSIR045 | AMSIR046 | AMSIR047 | AMSIR048 |
| 40 | AMSIR049 | AMSIR050 | AMSIR051 | AMSIR052 | AMSIR053 | AMSIR054 |
 Download Brochure
Download Brochure